Relationship of Pressure with Volume and Temperature
This video is a short explanation about the formulas of Gas law
Warning!!!Decrease your volume XD
Warning!!!Decrease your volume XD
Boyle's Law
for a given mass of gas at a constant temperature the volume of a gas varies inversely with the pressure
Example problem
A sample of gas occupies 7 L under a pressure of 2.2 atm. what would its volume be if the pressure were increased to 3.6 atm?*(assuming temp is constant)
Charle’s Law
at a constant pressure and for constant mass, the volume of a gas is directly proportional to the temperature.
Example problem
What is the final volume if a 20 L sample gas is heated from 25-degree Celsius to 50-degree Celsius?
(assuming pressure is constant)
Gay-Lussac’s law
Gay-Lussac accidentally discovered that at fixed volume and mass of a gas, the pressure of that gas is directly proportional to the temperature. This mathematically can be written as: p ∝ T
Example problem
The pressure in an automobile tire is 1.92 atm at 25-degree Celsius what will be the pressure if the temperature warms up to 37-degree Celsius?
Combined Gas law
The Combined gas law or General Gas Equation is obtained by combining Boyle's Law, Charles's law, and Gay-Lussac's Law. It shows the relationship between the pressure, volume, and temperature for a fixed mass (quantity) of gas:
Example problem
A helium balloon has a volume of 3 L at 25-degree Celsius and 1.08 atm find the final pressure of the if its final volume is 4.2 L at 10-degree Celsius?
Avogadro's law
Equal Volume of gases at the same temperature and pressure contains equal number of particles.
Example problem
If a 0.36 mol of argon gas occupies a volume of 7.62 ml, what volume would a 0.33 mol of argon have if they have the same temperature and pressure?
Given
argon argon
n=0.36 mol n=0.33 mol
V=7.62 ml V=?
I made this gif to explain that Avogadros law is the only Gas law that is not included to the
Combined gas law formula
Reference
https://www.toppr.com/guides/chemistry/states-of-matter/gas-laws/









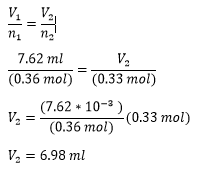


No comments:
Post a Comment